
Welcome to Ms. Stephens AP Environmental Science Class
Email: [email protected]
Class website: www.astephensscience.com
Tutorial: Wednesday or Thursday schedule an appointment.
Announcements:
The class website contains learning objectives, notes, labs and study links. Please check the website to see what you missed, and for make-up work.
Notice to Parents & Students:
The class website is regularly updated for each unit. Students are expected to check the class website prior to the next class when they are absent to not get behind. There are many study resources available as well!
Course Syllabus: APES Course syllabus Fall 2022
** Please download the Course Syllabus by clicking the link above and confirm you have read the course syllabus by completing the confirmation form on the google classroom**
Mission Possible at Maynard Jackson High School
Online Learning Expectations
Prezi of Class procedures and rules
Class Procedures Quiz
Online Multiple Intelligence Test- Save As... to save the graph of your results, print, email me, or save using copy ScreenShot and paste in a google doc.
Email: [email protected]
Class website: www.astephensscience.com
Tutorial: Wednesday or Thursday schedule an appointment.
Announcements:
The class website contains learning objectives, notes, labs and study links. Please check the website to see what you missed, and for make-up work.
Notice to Parents & Students:
The class website is regularly updated for each unit. Students are expected to check the class website prior to the next class when they are absent to not get behind. There are many study resources available as well!
Course Syllabus: APES Course syllabus Fall 2022
** Please download the Course Syllabus by clicking the link above and confirm you have read the course syllabus by completing the confirmation form on the google classroom**
Mission Possible at Maynard Jackson High School
Online Learning Expectations
Prezi of Class procedures and rules
Class Procedures Quiz
Online Multiple Intelligence Test- Save As... to save the graph of your results, print, email me, or save using copy ScreenShot and paste in a google doc.

Here is the link to the online Environmental Science Book Companion Site
Living in the Environment: Principles, Connections, and Solutions, 15th Edition
This link contains:
Living in the Environment: Principles, Connections, and Solutions, 15th Edition
This link contains:
- Chapter power points
- Chapter tests
- Concept maps
- Flashcards
- Glossaries
Classwork Assignments
Instructions and rubric for reading guides
Lab Report Rubric
One Small Thing Essay Ramen Article for OST Plastic Bottle Articl for OST
Week #1
Objectives: The objectives this week is to introduce students to the topics in AP Environmental Science and to introduce students to the structure, resources and pacing of the course. In addition, the first week activities include ice breakers, collaborative activities and reading/writing assessment. The main topic of this week is sustainability.
First Week: 1st Day of School : Mission Possible Prezi, Class Procedures quiz, Review class website.
Pre-teaching Activity: APES- Bingo game
Chapter 1 Lecture ppt
Chapter 1 Lecture guide questions
Watch/Write: The Story of Stuff- Video & Reflection
Class Activity: Ecological Footprint Assignment (computers)
Resources:
The Story of Stuff Website
Project: About me/One small thing
Video: Before the Flood
Video guide questions
Instructions and rubric for reading guides
Lab Report Rubric
One Small Thing Essay Ramen Article for OST Plastic Bottle Articl for OST
Week #1
Objectives: The objectives this week is to introduce students to the topics in AP Environmental Science and to introduce students to the structure, resources and pacing of the course. In addition, the first week activities include ice breakers, collaborative activities and reading/writing assessment. The main topic of this week is sustainability.
First Week: 1st Day of School : Mission Possible Prezi, Class Procedures quiz, Review class website.
Pre-teaching Activity: APES- Bingo game
Chapter 1 Lecture ppt
Chapter 1 Lecture guide questions
Watch/Write: The Story of Stuff- Video & Reflection
Class Activity: Ecological Footprint Assignment (computers)
Resources:
The Story of Stuff Website
Project: About me/One small thing
Video: Before the Flood
Video guide questions

Please read the course description below.
A copy of the Course Description from
the AP College Board is available here.
heCourse Objectives
Upon completion of the course, SWBAT:
Course Content Delivery and Teaching Strategies
In each unit, students learn the basic Environmental Science terminology, discuss natural and anthropogenic environmental issues, practice problems associated with these concepts, perform hands-on and/or virtual labs and analyze computer simulations. Students are assessed using a mixture of traditional testing (based on the AP format) and non-traditional methods. Labs and field experiences are an important part of this course. Students complete one lab activity or research for each unit. Students represent their lab findings mathematically and graphically. Students should keep a lab notebook of all assignments for the course.
FRQ stands for Free Response Question.
Look through the following presentation and read "How to Answer the Free Response Questions".
This very detailed document will help you understand the types of free response questions with tips for each type. FRQ questions are graded on a scale of 1-10.
APES Math Tutorial Video Part 1
APES Math Tutorial Video Part 2
Math in AP Environmental Science?
As you begin this course, you will hear the two words most dreaded by high school students...NO CALCULATORS! The AP College board test center will not allow you to access any tools for calculations on the May exam. Therefore, at no time during this course will you be able to use a calculator or any other tool to "get your answer." The good news is that most calculations on the tests and exams are written to be fairly easy calculations and to come out in whole numbers or to only a few decimal places. The challenge is in setting up the problems correctly and knowing enough basic math to solve the problems. With practice, you will be a math expert by the time the exam rolls around. So bid your calculator a fond farewell, tuck it away so you won't be tempted, and start sharpening your math skills!
A copy of the Course Description from
the AP College Board is available here.
heCourse Objectives
Upon completion of the course, SWBAT:
- Develop an understanding and appreciation for the earth's environment and the interrelationships of the natural world;
- Identify and analyze environmental problems, and examine multiple solutions for resolving and/or preventing them;
- Demonstrate the ability to apply computational scientific skills, including quantifiable data analysis;
- Develop collaborative relationships with others;
- Pass the APES exam with a score of 3 or better; and
- Enjoy the pace, academic challenges, and debate offered in the course.
Course Content Delivery and Teaching Strategies
In each unit, students learn the basic Environmental Science terminology, discuss natural and anthropogenic environmental issues, practice problems associated with these concepts, perform hands-on and/or virtual labs and analyze computer simulations. Students are assessed using a mixture of traditional testing (based on the AP format) and non-traditional methods. Labs and field experiences are an important part of this course. Students complete one lab activity or research for each unit. Students represent their lab findings mathematically and graphically. Students should keep a lab notebook of all assignments for the course.
FRQ stands for Free Response Question.
Look through the following presentation and read "How to Answer the Free Response Questions".
This very detailed document will help you understand the types of free response questions with tips for each type. FRQ questions are graded on a scale of 1-10.
APES Math Tutorial Video Part 1
APES Math Tutorial Video Part 2
Math in AP Environmental Science?
As you begin this course, you will hear the two words most dreaded by high school students...NO CALCULATORS! The AP College board test center will not allow you to access any tools for calculations on the May exam. Therefore, at no time during this course will you be able to use a calculator or any other tool to "get your answer." The good news is that most calculations on the tests and exams are written to be fairly easy calculations and to come out in whole numbers or to only a few decimal places. The challenge is in setting up the problems correctly and knowing enough basic math to solve the problems. With practice, you will be a math expert by the time the exam rolls around. So bid your calculator a fond farewell, tuck it away so you won't be tempted, and start sharpening your math skills!
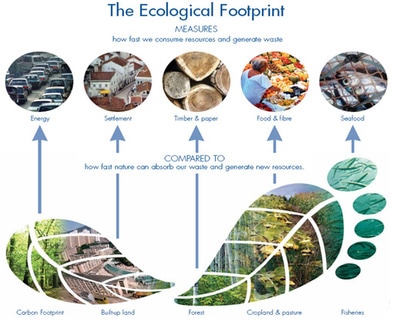
Unit 1: The Living World
AP Exam (10-15%)
Ecology and Earth Science Unit 1
Mr. Andersen Plate Tecs Video Guide Chaparral Food Chain
Plant Decomposition Lab Ecological Footprint Calculator
Ecological Footprint Info Biome Stations Activity Biome Map Biogeochemical Nutrient Cycle WS
Global Carbon Analysis Activity ANWR Debate Evidence
Primary Succession Diagram Secondary Succession Diagram
Tragedy of Commons Simulation Ecology Review Worksheet
Ecology Vocabulary PCB Last Frontier Inquiry
Ecology Road Map
Ecology and Earth Science Unit 1 PPTs
Ecology and Energy Lecture
Energy Transfer Lecture
Eagle Evidence PowerPoint
Plant Decomposition Prelab PPT
Species Diversity and Succession Lecture
Terrestrial Biomes Lecture
Niches and Interactions Between Species Lecture
Alaska: To Drill or Not to Drill Lecture
Homework Assignments for Unit 1
Summer HW 1: Environmental Disasters
Summer HW 2: Biomes
Summer HW 3: Earth Science Review
Summer HW 4: Math Problems!
Summer HW 5: Environmental Disease Research
8/24:
1) Have your parents go online and read the syllabus and complete the confirmation on the google classroom.
2) Write "My Summer" Practice Essay in AP style.
3) Sign up for Remind service.
8/25:
1) Ecological Footprint calculator and information.
2) Write the One Small Thing Essay
8/28: Do the Ecology Review Worksheet.
9/4: Due Ecology Study Card File Folder Assignment.
AP Exam (10-15%)
Ecology and Earth Science Unit 1
Mr. Andersen Plate Tecs Video Guide Chaparral Food Chain
Plant Decomposition Lab Ecological Footprint Calculator
Ecological Footprint Info Biome Stations Activity Biome Map Biogeochemical Nutrient Cycle WS
Global Carbon Analysis Activity ANWR Debate Evidence
Primary Succession Diagram Secondary Succession Diagram
Tragedy of Commons Simulation Ecology Review Worksheet
Ecology Vocabulary PCB Last Frontier Inquiry
Ecology Road Map
Ecology and Earth Science Unit 1 PPTs
Ecology and Energy Lecture
Energy Transfer Lecture
Eagle Evidence PowerPoint
Plant Decomposition Prelab PPT
Species Diversity and Succession Lecture
Terrestrial Biomes Lecture
Niches and Interactions Between Species Lecture
Alaska: To Drill or Not to Drill Lecture
Homework Assignments for Unit 1
Summer HW 1: Environmental Disasters
Summer HW 2: Biomes
Summer HW 3: Earth Science Review
Summer HW 4: Math Problems!
Summer HW 5: Environmental Disease Research
8/24:
1) Have your parents go online and read the syllabus and complete the confirmation on the google classroom.
2) Write "My Summer" Practice Essay in AP style.
3) Sign up for Remind service.
8/25:
1) Ecological Footprint calculator and information.
2) Write the One Small Thing Essay
8/28: Do the Ecology Review Worksheet.
9/4: Due Ecology Study Card File Folder Assignment.

Science, Matter, Energy, and Systems
Chapter 2
While energy flows in a complex, but ultimately one way path through nature, materials are endlessly recycled. The major material cycles (water, carbon, nitrogen, phosphorus, and sulfur) are critically important to living organisms. As humans interfere with these material cycles, we make it easier for some organisms to survive and more difficult for others. Often, we're intent on manipulating material cycles for our own short-term gain, but we don't think about the consequences for other species or even for ourselves in the long term. Clearly, it's important to understand these environmental systems and to take them into account in our public policy.
SWBAT :
Review how scientists use the scientific method IOT
study environmental phenomenon
Essential Questions
Class Demonstration: Light Re-light
Mankind: The story of us: Fire
Chapter 2
While energy flows in a complex, but ultimately one way path through nature, materials are endlessly recycled. The major material cycles (water, carbon, nitrogen, phosphorus, and sulfur) are critically important to living organisms. As humans interfere with these material cycles, we make it easier for some organisms to survive and more difficult for others. Often, we're intent on manipulating material cycles for our own short-term gain, but we don't think about the consequences for other species or even for ourselves in the long term. Clearly, it's important to understand these environmental systems and to take them into account in our public policy.
SWBAT :
Review how scientists use the scientific method IOT
study environmental phenomenon
Essential Questions
- How does carbon move through the carbon cycle?
- How have humans impacted the nitrogen cycle?
- Why is phosphorous not able to be transported to different locations during the phosphorous cycle?
- How are humans affecting the sulfur cycle?
- Why are phytoplankton such an important part of the ocean food chain?
Class Demonstration: Light Re-light
Mankind: The story of us: Fire

Ecosystems: What are they and how do they work?
Chapter 3
Eco Columns
Classroom Aquaculture and Hydroponic System Maintenance
The Globe program (students as citizen scientist)
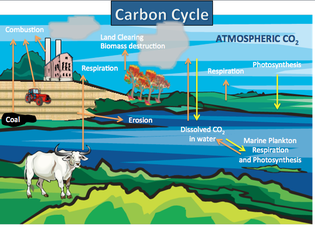
The Carbon Cycle
Carbon is essential to life as we know it. It is the basic building block of the carbohydrates, fats, proteins, DNA, and other organic compounds necessary to life.
Humans and other life forms require a balance of carbon dioxide in the atmosphere to maintain a moderate surface temperature. The carbon cycle provides this balance. Carbon dioxide in the air interacts with rain to form acid rain that reacts with rocks and is eventually deposited into the oceans. Sea creatures, like coral and clam, take the carbon out of the water to build their bodies/shells. When these sea creatures die, their bodies form limestone, which is eventually subducted under a continental plate and released again as volcanic gas. This process is part of the carbon cycle. No matter is created or destroyed in this process. It only changes form and location. Volcanoes release carbon dioxide (a greenhouse gas), which can raise the Earth's surface temperature. Volcanoes can also release ash, which can block the Sun's light from reaching Earth's surface, therefore decreasing the surface temperature.
Carbon in the atmosphere: Investigate changes
Assignment: Carbon Virtual Lab link here
Using this Carbon cycle simulator, you will experiment with how humans affect the cycle and what could happen between now and 2100. Pay attention to the atmospheric CO2 levels as you adjust the variables between the atmosphere, the biosphere, oceans, and the crust. This model is similar to models presented by the Intergovernmental Panel on Climate Change (IPCC). Between the years 1850 and 2006, atmospheric concentrations have risen from 290 parts per million (ppm) to over 380 ppm - a level higher than any known on Earth in more than 30 million years.
Carbon Cycle Lesson 1
Run the simulation to 2100 with the default settings, and, complete the Data Table, record the total carbon levels in each "sink" (terrestrial plants, soil, oil and gas, coal, Surface Ocean, and Deep Ocean) at 2050 and 2100.
Using the data you collect from the model, answer the following questions while thinking about how the model mimics real-life conditions:
To find out where all the carbon really goes, run the simulation again, one decade at a time. Record the total amount of carbon in the atmosphere (the number in the sky) and other carbon sinks (terrestrial plants, soil, surface ocean, and deep ocean), as carbon moves through the system. Note that 1 ppm of atmospheric CO2 is equivalent to 2.1 GT (Gigatons) of carbon. As you record your data, keep in mind that this is a simulation of real life. Answer the following questions:
Carbon is essential to life as we know it. It is the basic building block of the carbohydrates, fats, proteins, DNA, and other organic compounds necessary to life.
Humans and other life forms require a balance of carbon dioxide in the atmosphere to maintain a moderate surface temperature. The carbon cycle provides this balance. Carbon dioxide in the air interacts with rain to form acid rain that reacts with rocks and is eventually deposited into the oceans. Sea creatures, like coral and clam, take the carbon out of the water to build their bodies/shells. When these sea creatures die, their bodies form limestone, which is eventually subducted under a continental plate and released again as volcanic gas. This process is part of the carbon cycle. No matter is created or destroyed in this process. It only changes form and location. Volcanoes release carbon dioxide (a greenhouse gas), which can raise the Earth's surface temperature. Volcanoes can also release ash, which can block the Sun's light from reaching Earth's surface, therefore decreasing the surface temperature.
Carbon in the atmosphere: Investigate changes
Assignment: Carbon Virtual Lab link here
Using this Carbon cycle simulator, you will experiment with how humans affect the cycle and what could happen between now and 2100. Pay attention to the atmospheric CO2 levels as you adjust the variables between the atmosphere, the biosphere, oceans, and the crust. This model is similar to models presented by the Intergovernmental Panel on Climate Change (IPCC). Between the years 1850 and 2006, atmospheric concentrations have risen from 290 parts per million (ppm) to over 380 ppm - a level higher than any known on Earth in more than 30 million years.
Carbon Cycle Lesson 1
Run the simulation to 2100 with the default settings, and, complete the Data Table, record the total carbon levels in each "sink" (terrestrial plants, soil, oil and gas, coal, Surface Ocean, and Deep Ocean) at 2050 and 2100.
Using the data you collect from the model, answer the following questions while thinking about how the model mimics real-life conditions:
- If only one half of the flora in the world existed in 2100 (perhaps due to deforestation), what do you predict the atmospheric carbon level would be? How would you change the simulation to reflect this?
- What is the relationship between increased carbon in the ocean and increased carbon in the soil? How else might carbon be transferred to soil?
- Using the data generated by the simulation, determine the mathematical relationship between the percentage increase in fossil fuel consumption and the increase in atmospheric carbon. Is the relationship linear?
To find out where all the carbon really goes, run the simulation again, one decade at a time. Record the total amount of carbon in the atmosphere (the number in the sky) and other carbon sinks (terrestrial plants, soil, surface ocean, and deep ocean), as carbon moves through the system. Note that 1 ppm of atmospheric CO2 is equivalent to 2.1 GT (Gigatons) of carbon. As you record your data, keep in mind that this is a simulation of real life. Answer the following questions:
- What is the relationship between an increase in fossil fuel consumption and increased carbon in terrestrial plants? How might this change flora population? What impact could twenty years at this level of consumption have on flora?
- What is the relationship between an increase in total carbon concentration (the smokestack) and increased carbon in the ocean surface? How might this change marine life populations? What impact could fifty years at this level of emissions have on marine fauna? On marine flora?
- In addition to circulating through the carbon cycle, where else might excess carbon be found? In fifty years, where would you be most likely to see excess carbon?
- Which areas are most highly (and quickly) affected by an increase in carbon emissions (and increase in fossil fuel consumption)? How would these effects manifest themselves? What are the dangers/benefits to these areas?
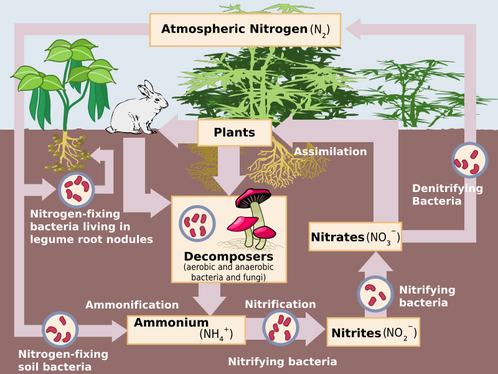
Nitrogen Cycle
Nitrogen and phosphorus are two of the most essential mineral nutrients for all types of ecosystems and often limit growth if they are not available in sufficient quantities. This is why the basic ingredients in plant fertilizer are nitrogen, phosphorus, and potassium, commonly abbreviated as NPK. A slightly expanded version of the basic equation for photosynthesis shows how plants use energy from the sun to turn nutrients and carbon into organic compounds:
Our atmosphere is composed of 78% N and 21% O. However, this atmospheric nitrogen cannot be used by organisms without some assistance. The nitrogen cycle is unique because it has stages during which bacteria help convert the nitrogen into useable forms. Nitrogen is essential for life because it helps to develop proteins, DNA, and RNA, and provide for plant growth. It can also be a limiting factor in plant growth.
The nitrogen cycle begins in the atmosphere as a gas N2 . It then goes through many steps throughout its cycle.
For it to be made useable by organisms, N2 needs to be "fixed". This can occur by lightning or with the help of nitrogen-fixing bacteria. Nitrogen-fixing bacteria live in the soil and in nodules on the roots of legumes.
Click here to watch an animation of nitrogen fixation.
When N2 is fixed, it's combined with hydrogen to form ammonia (NH3). This fixing process is called ammonification.
The water-soluble ion of NH3 is converted to (NH4+), which can be taken in by plants through their roots. NH4+ then goes through a process known as nitrification, in which it is converted into nitrite ions (NO2-) and then nitrate ions (NO3-). This process is conducted by specialized bacteria called nitrifying bacteria. These ions can be taken in by plants since they too, are water soluble.
The ammonium, nitrite, and nitrate ions can be assimilated and taken in by plants. Animals then receive nitrogen through consuming plants. Decomposers receive nitrogen through the decomposition of waste and decaying plants and animals. Decomposers process the nitrogen substances they take in and return the nitrogen to the soil as ammonium ions. Decomposition makes the nitrogen available to go through nitrification again. For nitrogen to return to the atmosphere in its gaseous N2 form, it must be denitrified. Denitrifying bacteria convert nitrates into N2+.
Humans have intervened in the nitrogen cycle by developing a way to fix nitrogen artificially, creating fertilizer. This fixation process, called the Haber process, or Haber-Bosch process conducted on an enormous scale, has negatively altered the nitrogen cycle by nearly doubling the amount of nitrogen fixation occurring on the Earth. Excess nitrogen in an aquatic environment can led to the eutrophication of the ecosystem. Fritz Haber, a German chemist, developed the process in the first decade of the 20th century. Karl Bosch, another German scientist, adapted the process for industrial use.
Nitrogen and phosphorus are two of the most essential mineral nutrients for all types of ecosystems and often limit growth if they are not available in sufficient quantities. This is why the basic ingredients in plant fertilizer are nitrogen, phosphorus, and potassium, commonly abbreviated as NPK. A slightly expanded version of the basic equation for photosynthesis shows how plants use energy from the sun to turn nutrients and carbon into organic compounds:
Our atmosphere is composed of 78% N and 21% O. However, this atmospheric nitrogen cannot be used by organisms without some assistance. The nitrogen cycle is unique because it has stages during which bacteria help convert the nitrogen into useable forms. Nitrogen is essential for life because it helps to develop proteins, DNA, and RNA, and provide for plant growth. It can also be a limiting factor in plant growth.
The nitrogen cycle begins in the atmosphere as a gas N2 . It then goes through many steps throughout its cycle.
For it to be made useable by organisms, N2 needs to be "fixed". This can occur by lightning or with the help of nitrogen-fixing bacteria. Nitrogen-fixing bacteria live in the soil and in nodules on the roots of legumes.
Click here to watch an animation of nitrogen fixation.
When N2 is fixed, it's combined with hydrogen to form ammonia (NH3). This fixing process is called ammonification.
The water-soluble ion of NH3 is converted to (NH4+), which can be taken in by plants through their roots. NH4+ then goes through a process known as nitrification, in which it is converted into nitrite ions (NO2-) and then nitrate ions (NO3-). This process is conducted by specialized bacteria called nitrifying bacteria. These ions can be taken in by plants since they too, are water soluble.
The ammonium, nitrite, and nitrate ions can be assimilated and taken in by plants. Animals then receive nitrogen through consuming plants. Decomposers receive nitrogen through the decomposition of waste and decaying plants and animals. Decomposers process the nitrogen substances they take in and return the nitrogen to the soil as ammonium ions. Decomposition makes the nitrogen available to go through nitrification again. For nitrogen to return to the atmosphere in its gaseous N2 form, it must be denitrified. Denitrifying bacteria convert nitrates into N2+.
Humans have intervened in the nitrogen cycle by developing a way to fix nitrogen artificially, creating fertilizer. This fixation process, called the Haber process, or Haber-Bosch process conducted on an enormous scale, has negatively altered the nitrogen cycle by nearly doubling the amount of nitrogen fixation occurring on the Earth. Excess nitrogen in an aquatic environment can led to the eutrophication of the ecosystem. Fritz Haber, a German chemist, developed the process in the first decade of the 20th century. Karl Bosch, another German scientist, adapted the process for industrial use.
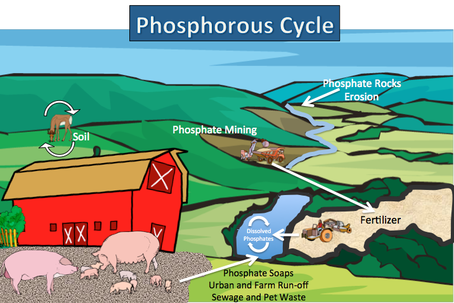
Phosphorous Cycle
The phosphorus cycle is probably the easiest of the biogeochemical cycles to describe. Phosphorus does not exist in the atmosphere except in dust particles. It is necessary for living organisms, as it is the backbone of nucleic acids (DNA and RNA) and other important biological molecules. Phosphorus tends to move through a local cycle, whereas the other cycles are global in nature, mostly because of the Earth's weather. Since phosphorus is limited in the atmosphere, it is unlikely to move great distances.
Phosphorus is found in soil, rock, and sediments. It is released from these rock forms through chemical weathering in the form of phosphate (PO4-3). Phosphate is highly soluble in aqueous solutions and can be absorbed from the soil into plants through their roots. Often phosphorus is a limiting factor for plant growth, as little of it is released into the environment.
Phosphorus can enter the water table and ultimately travel to the oceans, where it settles on the ocean floor. Later, through geological processes, ocean mixing, and upwelling, these rocks on the ocean floor may rise and become new land surface, with the result that their components can reenter the terrestrial cycles.
Humans affect the phosphorus cycle by mining phosphorus-rich rocks for the purpose of processing them and adding them to commercial inorganic fertilizers. The phosphorus is easily leached into the groundwater and can find its way into aquatic ecosystems; where it can help promote algae and other aquatic plant growth than can lead to overgrowth of these plants and ultimately eutrophication of the pond or lake. Phosphorus also can be added to ecosystems by humans through the release of untreated sewage, agricultural runoff, and detergents.
The phosphorus cycle is probably the easiest of the biogeochemical cycles to describe. Phosphorus does not exist in the atmosphere except in dust particles. It is necessary for living organisms, as it is the backbone of nucleic acids (DNA and RNA) and other important biological molecules. Phosphorus tends to move through a local cycle, whereas the other cycles are global in nature, mostly because of the Earth's weather. Since phosphorus is limited in the atmosphere, it is unlikely to move great distances.
Phosphorus is found in soil, rock, and sediments. It is released from these rock forms through chemical weathering in the form of phosphate (PO4-3). Phosphate is highly soluble in aqueous solutions and can be absorbed from the soil into plants through their roots. Often phosphorus is a limiting factor for plant growth, as little of it is released into the environment.
Phosphorus can enter the water table and ultimately travel to the oceans, where it settles on the ocean floor. Later, through geological processes, ocean mixing, and upwelling, these rocks on the ocean floor may rise and become new land surface, with the result that their components can reenter the terrestrial cycles.
Humans affect the phosphorus cycle by mining phosphorus-rich rocks for the purpose of processing them and adding them to commercial inorganic fertilizers. The phosphorus is easily leached into the groundwater and can find its way into aquatic ecosystems; where it can help promote algae and other aquatic plant growth than can lead to overgrowth of these plants and ultimately eutrophication of the pond or lake. Phosphorus also can be added to ecosystems by humans through the release of untreated sewage, agricultural runoff, and detergents.
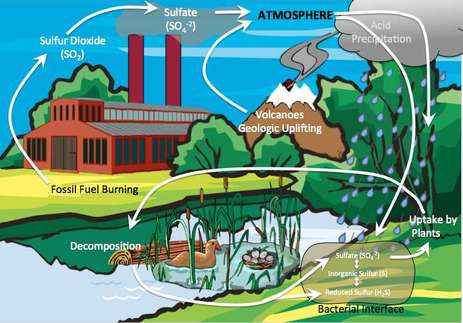
Sulfur Cycle
An important component of protein and vitamins, sulfur is essential for plant and animal health. Naturally, most sulfur is located in rocks and ocean sediments, but some is also found in the atmosphere.
The natural sulfur cycle is described by the following path:
The U.S. is no longer the top energy consumer according to the International Energy Agency. This occurred in 2009 when China rose to the global energy apex, consuming 2,252 billion tons of oil equivalent energy from sources such as coal, nuclear power, natural gas and hydro-electric power—about 4 percent more than the United States. In 2000, China only consumed half of the energy of the USA. Demand for energy is soaring as Chinese are buying more cars, moving into bigger houses that need more heat, purchasing more televisions, refrigerators and other appliances and starting new businesses and factories.
Sulfur is different than other minerals with respect to mining issues and supply concerns. As long as the world economy is petroleum-driven, the sustainable production of necessary sulfur supplies is not in question. Instead of relying on sulfur mining, it is possible to recover enough sulfur for the global demand using the natural byproducts at natural gas plants and petroleum refineries. Byproduct H2SO4 from metal smelters contributes additional supplies.
While the production of recovered sulfur has been increasing around the world as a result of growing environmental awareness and concerns, the use of sulfur in a multitude of end uses has increased at a slower pace. Huge quantities of sulfur and H2SO4 are consumed in many industries, but not as much as is produced. Expansive world trade transfers large quantities of sulfur from the large producing areas to the large consumers. Stocks, however, are accumulating at recovery operations in remote locations where the distances to the market make its transportation prohibitively expensive. In such countries as Kazakhstan, the infrastructure is inadequate for transporting the large volumes of sulfur produced.
The cumulative effects of sulfur production also differ from the results of other mineral production. Modern sulfur production actually results in improved environmental conditions rather than mining processes and outcomes that must be repaired. Sulfur recovery prevents the emissions of SO2 and other harmful compounds into the atmosphere, avoiding the detrimental effects of those emissions.
The challenges facing the sulfur industry are unique. In other mineral industries, the major concerns are how to continue to produce necessary materials and protect the environment as much as possible at the same time. The sulfur industry is confronting the question of what to do with all the sulfur it produces. Current production exceeds consumption by a relatively small percentage, but environmental regulations continue to increase with little growth in sulfur uses. The 21st century sulfur industry will need to expand sulfur consumption in nontraditional markets and find acceptable ways to dispose of unneeded sulfur without compromising environmental protection.
An important component of protein and vitamins, sulfur is essential for plant and animal health. Naturally, most sulfur is located in rocks and ocean sediments, but some is also found in the atmosphere.
The natural sulfur cycle is described by the following path:
- Sulfur is naturally released into the atmosphere from rocks and sediment in the forms of hydrogen sulfide (H2S) and sulfur dioxide (SO2) through weathering, volcanic eruptions, and the decay of dead organisms.
- Once in the atmosphere, SO2 reacts with oxygen to form sulfur trioxide (SO3) and with water to form sulfuric acid (H2SO4)
- Sulfur particles are deposited back into the soil and water, or they combine with water and fall in the form of acid precipitation.
- Plants absorb sulfate ions (SO42-) through their roots, and animals receive sulfur by consuming plants.
- Burning sulfur-containing coal and oil to produce electric power, producing about two-thirds of the human inputs of sulfur dioxide.
- Refining sulfur-containing petroleum to make gasoline, heating oil, and other useful products.
- Using smelting to convert sulfur compounds of metallic minerals into free metals such as copper, lead, and zinc.
The U.S. is no longer the top energy consumer according to the International Energy Agency. This occurred in 2009 when China rose to the global energy apex, consuming 2,252 billion tons of oil equivalent energy from sources such as coal, nuclear power, natural gas and hydro-electric power—about 4 percent more than the United States. In 2000, China only consumed half of the energy of the USA. Demand for energy is soaring as Chinese are buying more cars, moving into bigger houses that need more heat, purchasing more televisions, refrigerators and other appliances and starting new businesses and factories.
Sulfur is different than other minerals with respect to mining issues and supply concerns. As long as the world economy is petroleum-driven, the sustainable production of necessary sulfur supplies is not in question. Instead of relying on sulfur mining, it is possible to recover enough sulfur for the global demand using the natural byproducts at natural gas plants and petroleum refineries. Byproduct H2SO4 from metal smelters contributes additional supplies.
While the production of recovered sulfur has been increasing around the world as a result of growing environmental awareness and concerns, the use of sulfur in a multitude of end uses has increased at a slower pace. Huge quantities of sulfur and H2SO4 are consumed in many industries, but not as much as is produced. Expansive world trade transfers large quantities of sulfur from the large producing areas to the large consumers. Stocks, however, are accumulating at recovery operations in remote locations where the distances to the market make its transportation prohibitively expensive. In such countries as Kazakhstan, the infrastructure is inadequate for transporting the large volumes of sulfur produced.
The cumulative effects of sulfur production also differ from the results of other mineral production. Modern sulfur production actually results in improved environmental conditions rather than mining processes and outcomes that must be repaired. Sulfur recovery prevents the emissions of SO2 and other harmful compounds into the atmosphere, avoiding the detrimental effects of those emissions.
The challenges facing the sulfur industry are unique. In other mineral industries, the major concerns are how to continue to produce necessary materials and protect the environment as much as possible at the same time. The sulfur industry is confronting the question of what to do with all the sulfur it produces. Current production exceeds consumption by a relatively small percentage, but environmental regulations continue to increase with little growth in sulfur uses. The 21st century sulfur industry will need to expand sulfur consumption in nontraditional markets and find acceptable ways to dispose of unneeded sulfur without compromising environmental protection.
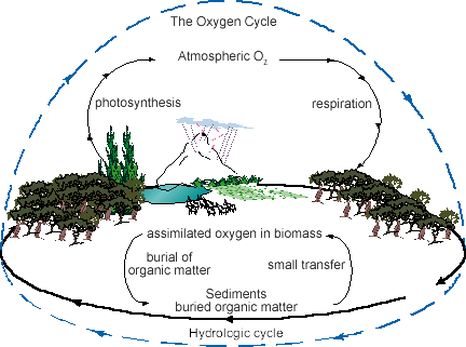
Oxygen Cycle
The Oxygen cycle is not described in most textbooks but is nevertheless very important for the living world. Oxygen (O2) is the byproduct of photosynthesis in plants and a reactant in the cellular respiration of plants and animals. Oxygen is the second major component of the atmosphere after nitrogen gas (N2) and composes approximately 21 percent of the atmosphere.
Oxygen is a reactive molecule. Besides involvement in the photosynthetic and cellular respiration reactions, oxygen is removed from the atmosphere during the weathering process of rock and minerals. As new rocks and minerals are exposed to the atmosphere during weathering, oxygen combines with them in a process called oxidation and is, thus, removed from the atmosphere.
Oxygen is also important in the formation of atmospheric ozone. Sunlight breaks water (H2O) vapor into hydrogen gas and oxygen, and the hydrogen escapes Earth's atmosphere and travels into outer space. Ozone (O3) is a naturally occurring reaction product in the atmosphere, where it forms a layer. The ozone layer is important for filtering out much of the harmful ultraviolet (UV) radiation from the sun and keeping it from hitting the Earth.
The chemical reaction for the formation of ozone is:
The Oxygen cycle is not described in most textbooks but is nevertheless very important for the living world. Oxygen (O2) is the byproduct of photosynthesis in plants and a reactant in the cellular respiration of plants and animals. Oxygen is the second major component of the atmosphere after nitrogen gas (N2) and composes approximately 21 percent of the atmosphere.
Oxygen is a reactive molecule. Besides involvement in the photosynthetic and cellular respiration reactions, oxygen is removed from the atmosphere during the weathering process of rock and minerals. As new rocks and minerals are exposed to the atmosphere during weathering, oxygen combines with them in a process called oxidation and is, thus, removed from the atmosphere.
Oxygen is also important in the formation of atmospheric ozone. Sunlight breaks water (H2O) vapor into hydrogen gas and oxygen, and the hydrogen escapes Earth's atmosphere and travels into outer space. Ozone (O3) is a naturally occurring reaction product in the atmosphere, where it forms a layer. The ozone layer is important for filtering out much of the harmful ultraviolet (UV) radiation from the sun and keeping it from hitting the Earth.
The chemical reaction for the formation of ozone is:
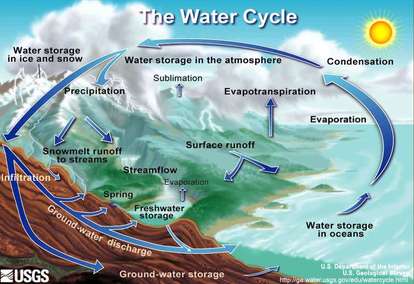
The Hydrologic Cycle
The water cycle is one of the first elementary science topics introduced to young children. As one moves from elementary, middle to high school, we begin to understand the complexity and integration of the hydrologic cycle with the other biogeochemical cycles.
Basically, it is understood that:
Precipitation: Any form of water falling from the atmosphere to the ground.
The water cycle is one of the first elementary science topics introduced to young children. As one moves from elementary, middle to high school, we begin to understand the complexity and integration of the hydrologic cycle with the other biogeochemical cycles.
Basically, it is understood that:
- Water enters the atmosphere by evaporation and by transpiration from leaves.
- It condenses and falls from the atmosphere as precipitation.
- When water falls as precipitation on land, it has two possible pathways:
- Returns to the hydrosphere by flowing as runoff from the land surface into streams, rivers, lakes, and eventually the ocean.
- Returns to the lithosphere by infiltration into the ground becoming soil water or ground water
Precipitation: Any form of water falling from the atmosphere to the ground.
- Condensation: The process of changing from a gas to a liquid
- Evapotranspiration: Evaporation + transpiration = Evapotranspiration
- Evaporation: the transformation of water from liquid to gas phases as it moves from the ground or bodies of water into the overlying atmosphere.
- The source of energy for evaporation is primarily solar radiation
- Transpiration: the release of water vapor from plants into the atmosphere.
- Evaporation: the transformation of water from liquid to gas phases as it moves from the ground or bodies of water into the overlying atmosphere.
- Run-off: Variety of ways water moves across the land
- Snowmelt: run off from melting of snow on mountain tops
- Water can flow to rivers, lakes, reservoirs, oceans or infiltrate into soil
- Infiltration: Flow of water from surface into the ground
- Once in ground can become soil water or ground water


Essential Questions
DO NOW
Environmental richness is used to describe the variety and interconnectedness of life on Earth. It includes genetic diversity, species diversity, and ecosystem diversity around the world. The potential location of biological communities is determined in large part by climate, moisture availability, soil type, geomorphology and other natural features. Understanding the global distribution of biomes, and knowing the differences in who lives where and why, are essential to the study of global environmental science. Human occupation and use of natural resources is strongly dependent on the biomes found in particular locations. Natural selection, in which beneficial traits are passed from survivors to their progeny, is the mechanism by which evolution occurs. Species interactions-competition, predation, symbiosis, etc., are important factors in natural selection. Human introduction of new species as well as removal of existing ones can cause profound changes in biological communities and can compromise the life-supporting ecological services on which we all depend. Write a one paragraph reflection. Cite examples.
- What is the inherent value of biological diversity?
- How does the conservation of biological diversity involve an understanding of the intricate relationships among species and between species and their environments?
- What are the ecological functions of biological diversity?
- What major problems are associated with biological diversity and what impact does human activity have?
- How do species interact?
- What limits the growth of populations?
- How do communities and ecosystems respond to changing environmental conditions?
- How do species replace one another in ecological succession?
- Why should we protect certain species and not others?
DO NOW
Environmental richness is used to describe the variety and interconnectedness of life on Earth. It includes genetic diversity, species diversity, and ecosystem diversity around the world. The potential location of biological communities is determined in large part by climate, moisture availability, soil type, geomorphology and other natural features. Understanding the global distribution of biomes, and knowing the differences in who lives where and why, are essential to the study of global environmental science. Human occupation and use of natural resources is strongly dependent on the biomes found in particular locations. Natural selection, in which beneficial traits are passed from survivors to their progeny, is the mechanism by which evolution occurs. Species interactions-competition, predation, symbiosis, etc., are important factors in natural selection. Human introduction of new species as well as removal of existing ones can cause profound changes in biological communities and can compromise the life-supporting ecological services on which we all depend. Write a one paragraph reflection. Cite examples.
DO NOW
View Time The Fragile Earth images
Select one slide that you feel best summarized the title (Fragile Earth). Explain why you picked this slide.
View Time The Fragile Earth images
Select one slide that you feel best summarized the title (Fragile Earth). Explain why you picked this slide.
Ecosystem Webquest Project site
Chapter 5/6 notes
Biodiversity
Blackfish Writing Assignment Cane Toads Video Guide
Measuring Campus Biodiversity Lab Endangered Species Research Paper
Endangered Species Research Paper Ex Grizzly Man Video Guide
End Species Conservation Plan Nowhere to Roam Article and Questions
Biodiversity Vocabulary
Biodiversity Unit Road Map
Chapter 5/6 notes
Biodiversity
Blackfish Writing Assignment Cane Toads Video Guide
Measuring Campus Biodiversity Lab Endangered Species Research Paper
Endangered Species Research Paper Ex Grizzly Man Video Guide
End Species Conservation Plan Nowhere to Roam Article and Questions
Biodiversity Vocabulary
Biodiversity Unit Road Map

Populations:lations 2
Age Structure Diagram Analysis Popular Pyramids: USA Rwanda Activity
China's 1 Child Inquiry Discussion Population Math Equations
Population Math Problems I Population Math Problems II
Human Growth Web Exercise Activity World Population Growth Introduction
Population Density Inquiry Lab Tag and Recapture Lab
Population Review Guide Urban Sprawl Build Your Own City Project
China's Lost Girls Video Guide
Diseases Spread Through Population Readings and Discussion
HTPA Cemetery Activity: Survivorship
HTPA Cemetery Tombstones
Populations Vocabulary
Population Road Map
Population Unit 2 PPTs
Population Basics PPT
Human Population Basics PPT
US Population Info PPT
Urban Sprawl PPT
Homework for the unit:
10/3: Do the Ch 5 Reading Guide (Last Half of Chapter). Due on Tues 10/13.
10/17: Do Chapter 6 Reading Guide and do the White Nose Bat Assignment. Due on Tues 10/24.
10/24: Do Ch 22 Reading Guide. Due on Tues 10/31. Here is the Urban Sprawl Project Information and Rubric.
Presentations begin on Thur 11/2.
11/7: Do the Population Review Guide and the Population File Folder. Both due on Thursday 11/14.
11/16: Test Thursday on Populations. Complete your Population File Folder and Pop. Review Guide. See (11/7) post for links.
On Thursday, you will collect data from your lab. Construct a lab report that is due on Tues 11/28. Here is the lab report rubric.
APES Semester 1 Review Packet
Age Structure Diagram Analysis Popular Pyramids: USA Rwanda Activity
China's 1 Child Inquiry Discussion Population Math Equations
Population Math Problems I Population Math Problems II
Human Growth Web Exercise Activity World Population Growth Introduction
Population Density Inquiry Lab Tag and Recapture Lab
Population Review Guide Urban Sprawl Build Your Own City Project
China's Lost Girls Video Guide
Diseases Spread Through Population Readings and Discussion
HTPA Cemetery Activity: Survivorship
HTPA Cemetery Tombstones
Populations Vocabulary
Population Road Map
Population Unit 2 PPTs
Population Basics PPT
Human Population Basics PPT
US Population Info PPT
Urban Sprawl PPT
Homework for the unit:
10/3: Do the Ch 5 Reading Guide (Last Half of Chapter). Due on Tues 10/13.
10/17: Do Chapter 6 Reading Guide and do the White Nose Bat Assignment. Due on Tues 10/24.
10/24: Do Ch 22 Reading Guide. Due on Tues 10/31. Here is the Urban Sprawl Project Information and Rubric.
Presentations begin on Thur 11/2.
11/7: Do the Population Review Guide and the Population File Folder. Both due on Thursday 11/14.
11/16: Test Thursday on Populations. Complete your Population File Folder and Pop. Review Guide. See (11/7) post for links.
On Thursday, you will collect data from your lab. Construct a lab report that is due on Tues 11/28. Here is the lab report rubric.
APES Semester 1 Review Packet

Chapter 8/9: Population Ecology and Human Impacts
Learning goals:
1. I can identify issues occurring in various countries (India, Japan, United States) due to population growth or decline.
2. I can identify trends in population growth and the underlying reasons for the trends.
3. I can create an age structure diagram and the factors that change the structure of an age structure diagram.
4. I can create an demographic transition graph and describe the factors associated with each phase.
5.I can identify trends in global urbanization and the impacts economically and environmentally of these choices.
Chapter 8/9 ppt
Chapter 8/9 assignments
Videos:
All alone in the night sky
A View from Space
Don't Panic
The World in Balance
The World Bank population data
I Th e can identify trends in population growth and the underlying reasons for the trends

Water Unit
Water 3
Water, Water Everywhere Research
Gulf Of Mexico PPT
Watershed Diagram Huell Howser Desalinization Video Guide
Oligotrophic vs Eutrophic Lakes Diagram Ocean Zones Diagram
MTBE and You Information Leaky Faucet Activity
How Urbanization Affects Water Cycle Info Household Water Usage Calculations
Coastal Debate Activity Hoover Dam- Megastructures Video Guide
Great Wall Across Yangtze Video Guide Fisheries Research
Pacific Garbage Patch Info and WS Clean Water Act Research Paper
Mercury Rising Article from Time Water Testing Lab with Stations
Effect of Sewage on DO Levels Lab Water Treatment Flow Chart
Wastewater Treatment Video Guide Water Review Guide
Water Pollution Scenarios Stations Activity Vanishing midwest ogallala aquifer drought
Water Vocabulary
Water Unit Road Map
Water Unit 3 PPTs
Water Introduction PPT
Human Usage of Water PPT
Water Pollution Types PPT
Water Monitoring and Eutrophication PPT
Coastal Pollution and Water Treatment PPT
Water Testing Lab PPT
Homework for the Unit:
12/5: Do Chapter 8 Reading Guide. Due on Tuesday 12/12.
12/12: Due Ch 13 Reading Guide and Read and answer the questions for the MTBE article. Both are due on Tues 12/14.
Christmas break Assignments: Do Chapter 20 Reading Guide HW. Due on Thurs 1/4. Also, finish your eutrophication flow map. And, complete your water testing lab report. Here is the lab report rubric.
1/9: Do the APES Final Study Guide. Also, do the Water Folder Quick Study Guide, Due on Tues 1/16. Make sure to finish your Water Treatment Lab paper. Your unit exam is on Thursday 1/18.
Water 3
Water, Water Everywhere Research
Gulf Of Mexico PPT
Watershed Diagram Huell Howser Desalinization Video Guide
Oligotrophic vs Eutrophic Lakes Diagram Ocean Zones Diagram
MTBE and You Information Leaky Faucet Activity
How Urbanization Affects Water Cycle Info Household Water Usage Calculations
Coastal Debate Activity Hoover Dam- Megastructures Video Guide
Great Wall Across Yangtze Video Guide Fisheries Research
Pacific Garbage Patch Info and WS Clean Water Act Research Paper
Mercury Rising Article from Time Water Testing Lab with Stations
Effect of Sewage on DO Levels Lab Water Treatment Flow Chart
Wastewater Treatment Video Guide Water Review Guide
Water Pollution Scenarios Stations Activity Vanishing midwest ogallala aquifer drought
Water Vocabulary
Water Unit Road Map
Water Unit 3 PPTs
Water Introduction PPT
Human Usage of Water PPT
Water Pollution Types PPT
Water Monitoring and Eutrophication PPT
Coastal Pollution and Water Treatment PPT
Water Testing Lab PPT
Homework for the Unit:
12/5: Do Chapter 8 Reading Guide. Due on Tuesday 12/12.
12/12: Due Ch 13 Reading Guide and Read and answer the questions for the MTBE article. Both are due on Tues 12/14.
Christmas break Assignments: Do Chapter 20 Reading Guide HW. Due on Thurs 1/4. Also, finish your eutrophication flow map. And, complete your water testing lab report. Here is the lab report rubric.
1/9: Do the APES Final Study Guide. Also, do the Water Folder Quick Study Guide, Due on Tues 1/16. Make sure to finish your Water Treatment Lab paper. Your unit exam is on Thursday 1/18.

Air 4
Creating Acid Rain Lab Air Pollution Presentations
Cap and Trade Information Car Emissions Activity
Indoor Air Pollution Information Monitoring Air Pollution Lab
NAAQS Air Pollution Standards Secondary Pollutants Cheat Sheet
Schonbein Number Chart for Ozone Air Pollutants Review True/False
El Nino Information and Activity Air Unit Review
Emissions Trading Game
Air Vocabulary
Air Unit Road Map
Air Pollution Data Lab- EPA graphs
Homework for the unit:
1/23 Staple together.
1) Research the Dakota Access Pipeline Issue. And
2) watch the movie Dirt! and answer the questions in the video guide.
1/30: Write a 1-2 pg persuasive typed essay (double spaced, 12 point font) on "Should we farm fish?" Include MLA citation for all evidence used. Make sure to have an introduction and conclusion. Due on Tues 2/6. Don't forget to bring your Fisheries EdPuzzle back completed on Thursday 2/8.
2/13: Air Pollutant Presentations are due on Tues 2/20. Groups of 5 and no repeating air pollutant presentations. Also, do Ch 18 Part One Reading Guide. Due on Tuesday as well. Fisheries quiz postponed until Tues 2/27.
2/27: Do Chapter 18 Reading Guide Part Two and the Lake Nyos Case Study. Due Tues 3/6
3/6: Do the AQI Index Research and complete your lab report on Air Testing Lab. Due Tues 3/13.
3/15: Air Test on Thursday. Multiple Choice and FRQ. Here is the Air Quick Study Card due on Thursday 3/15.
Creating Acid Rain Lab Air Pollution Presentations
Cap and Trade Information Car Emissions Activity
Indoor Air Pollution Information Monitoring Air Pollution Lab
NAAQS Air Pollution Standards Secondary Pollutants Cheat Sheet
Schonbein Number Chart for Ozone Air Pollutants Review True/False
El Nino Information and Activity Air Unit Review
Emissions Trading Game
Air Vocabulary
Air Unit Road Map
Air Pollution Data Lab- EPA graphs
Homework for the unit:
1/23 Staple together.
1) Research the Dakota Access Pipeline Issue. And
2) watch the movie Dirt! and answer the questions in the video guide.
1/30: Write a 1-2 pg persuasive typed essay (double spaced, 12 point font) on "Should we farm fish?" Include MLA citation for all evidence used. Make sure to have an introduction and conclusion. Due on Tues 2/6. Don't forget to bring your Fisheries EdPuzzle back completed on Thursday 2/8.
2/13: Air Pollutant Presentations are due on Tues 2/20. Groups of 5 and no repeating air pollutant presentations. Also, do Ch 18 Part One Reading Guide. Due on Tuesday as well. Fisheries quiz postponed until Tues 2/27.
2/27: Do Chapter 18 Reading Guide Part Two and the Lake Nyos Case Study. Due Tues 3/6
3/6: Do the AQI Index Research and complete your lab report on Air Testing Lab. Due Tues 3/13.
3/15: Air Test on Thursday. Multiple Choice and FRQ. Here is the Air Quick Study Card due on Thursday 3/15.

Land Unit 5
Bhopal Article and Questions Food Inc Video Guide
GMOs: Benefits and Disadvantages Forest Fires in Idaho Worksheet
Land Agency Jigsaw Activity Coal Miner Flow Map
Cyanide Heap Leaching Diagram Mission Mining Activity
Pesticide Take Home Essay Pesticide Take Home Essay Rubric
Decomposition Inquiry Lab Soil Texture Diagram
Salinization Inquiry Lab Decomposition Intro Activity
Garbage Mountain Video Guide Land Review
Land Vocabulary
Land Unit Road Map
Homework for the unit:
3/20: Do Chapter 19 Part One Reading Guide. Due Tues 3/27.
3/27: Read the Climate Denier's Technique Article. Do the assignment at the end of the article. Due on 3/30.
Spring Break Assignments : All assignments Due 4/10 2. Finish the Interactive Farming Activity.
3. Do Ch 14 Reading Guide.
4. Do the Malnutrition Reading and table.
5.Do the Land Agency Reading and Assignment.

Energy 6
Fracking Info and Questions Keystone Pipeline Info and Questions
Oil Exploration Reading and Qstns Energy Math Intro Activity
Energy Math Collaborative Instructions Energy Math Probs I
Energy Math Probs II Yucca Mountain Article and Higher Level Qstns
Back to Chernobyl Video Guide Renewable Energy Presentations
Fuel Cell Information Guide Energy Review Guide
Energy Vocabulary Sell Me That Car! Activity
Shark Tank Energy Project
Oiled Feather Lab Activity

Global Concerns 7
Global Warming Effects Station Activity Inconvenient Truth Video Guide
Dimming the Sun Nova Video Guide Kyoto Protocol Information
Kyoto Protocol Assignment CO2 Reduction Tips
Montreal Protocol Information
Noise Pollution Decibel Activity Decibel Hell Article Reading
Tracking Buoy Data
Global Concerns Vocabulary
Global Unit Road Map
Homework for the unit:
4/10: Research an endangered species and create a poster with the information learned. Here are the guidelines. Due on Tuesday 4/17. We will do a gallery walk then so make sure to study your species.
Global Warming Effects Station Activity Inconvenient Truth Video Guide
Dimming the Sun Nova Video Guide Kyoto Protocol Information
Kyoto Protocol Assignment CO2 Reduction Tips
Montreal Protocol Information
Noise Pollution Decibel Activity Decibel Hell Article Reading
Tracking Buoy Data
Global Concerns Vocabulary
Global Unit Road Map
Homework for the unit:
4/10: Research an endangered species and create a poster with the information learned. Here are the guidelines. Due on Tuesday 4/17. We will do a gallery walk then so make sure to study your species.

APES Exam Review
APES Summative Assessment Review Assignment DUE April 24, 2017
Bozeman Science Ed Puzzles
Bozeman Science Review Videos
AP Environmental Science Video Review
Homework for the unit:
4/11: Study for your Mock Test MC an FRQ on Tues 4/18.
Here is the link to the Google Drive.
4/27: STUDY FOR THE AP TEST ON Thursday MAY 10.
5/15: National Park Project. Presentations start on Tuesday 5/22.
5/22: Bring your textbooks in.


